Complete the sentences about heme, a molecule of profound importance in biological systems. This intricate compound, composed of an iron ion nestled within a porphyrin ring, plays a pivotal role in a myriad of cellular processes, ranging from oxygen transport to signal transduction.
Throughout this comprehensive guide, we will delve into the intricate structure of heme, tracing its biosynthesis and degradation pathways. We will explore its interactions with proteins, unraveling the mechanisms by which it modulates their function and stability. Furthermore, we will shed light on the crucial role of heme in oxygen transport and metabolism, energy production, and cellular signaling.
Heme Structure and Composition
Heme is an essential cofactor for various proteins involved in oxygen transport, electron transfer, and enzymatic catalysis. It consists of a porphyrin ring, a planar structure composed of four pyrrole rings linked by methine bridges, and an iron ion (Fe 2+) at its center.
The porphyrin ring provides a stable environment for the iron ion, preventing its oxidation to Fe 3+. The iron ion is coordinated to the four nitrogen atoms of the porphyrin ring in a square planar geometry. Additionally, two axial ligands bind to the iron ion perpendicular to the porphyrin plane.
These axial ligands can be various molecules, such as water, oxygen, or nitrogenous ligands like histidine or imidazole.
Factors Affecting Heme Stability and Reactivity
The stability and reactivity of heme are influenced by several factors, including the nature of the axial ligands, the redox state of the iron ion, and the surrounding protein environment. The axial ligands can modulate the electronic properties of the iron ion, affecting its reactivity and oxygen affinity.
The redox state of the iron ion also plays a crucial role, with Fe 2+being more reactive and oxygen-binding than Fe 3+. Finally, the protein environment can influence heme’s stability and reactivity by providing a specific orientation and electrostatic interactions that affect its interactions with other molecules.
Heme Biosynthesis
Heme biosynthesis is a complex process that begins with the amino acid glycine and the coenzyme succinyl-CoA. The pathway involves a series of enzymatic reactions that take place in different compartments of the cell, including the mitochondria, cytoplasm, and endoplasmic reticulum.
Key Steps in Heme Biosynthesis
- Condensation of glycine and succinyl-CoA:This reaction is catalyzed by the enzyme aminolevulinic acid synthase (ALAS) and results in the formation of 5-aminolevulinic acid (ALA).
- Condensation of two ALA molecules:This reaction is catalyzed by the enzyme porphobilinogen synthase (PBGS) and results in the formation of porphobilinogen (PBG).
- Cyclization of four PBG molecules:This reaction is catalyzed by the enzyme hydroxymethylbilane synthase (HMBS) and results in the formation of hydroxymethylbilane (HMB).
- Isomerization and cyclization of HMB:This reaction is catalyzed by the enzyme uroporphyrinogen synthase (UROS) and results in the formation of uroporphyrinogen III.
- Decarboxylation and cyclization of uroporphyrinogen III:This reaction is catalyzed by the enzyme coproporphyrinogen oxidase (CPO) and results in the formation of coproporphyrinogen III.
- Oxidation of coproporphyrinogen III:This reaction is catalyzed by the enzyme protoporphyrinogen oxidase (PPO) and results in the formation of protoporphyrinogen IX.
- Insertion of iron into protoporphyrinogen IX:This reaction is catalyzed by the enzyme ferrochelatase and results in the formation of heme.
Regulation of Heme Biosynthesis
Heme biosynthesis is a highly regulated process that is essential for cellular metabolism. The rate of heme synthesis is controlled by a number of factors, including the availability of iron, the activity of the enzyme ALAS, and the levels of heme in the cell.
When iron is available, ALAS is activated and heme synthesis is increased. Conversely, when iron is not available, ALAS is inhibited and heme synthesis is decreased.
The levels of heme in the cell are also feedback-regulated. When the levels of heme are high, the activity of ALAS is inhibited. Conversely, when the levels of heme are low, the activity of ALAS is increased.
Disorders Associated with Impaired Heme Biosynthesis, Complete the sentences about heme
Impaired heme biosynthesis can lead to a number of disorders, including:
- Porphyrias:These are a group of disorders that are caused by defects in the enzymes involved in heme biosynthesis. Porphyrias can cause a variety of symptoms, including abdominal pain, nausea, vomiting, and skin photosensitivity.
- Sideroblastic anemias:These are a group of disorders that are caused by defects in the iron-sulfur cluster assembly machinery. Sideroblastic anemias can lead to anemia, fatigue, and weakness.
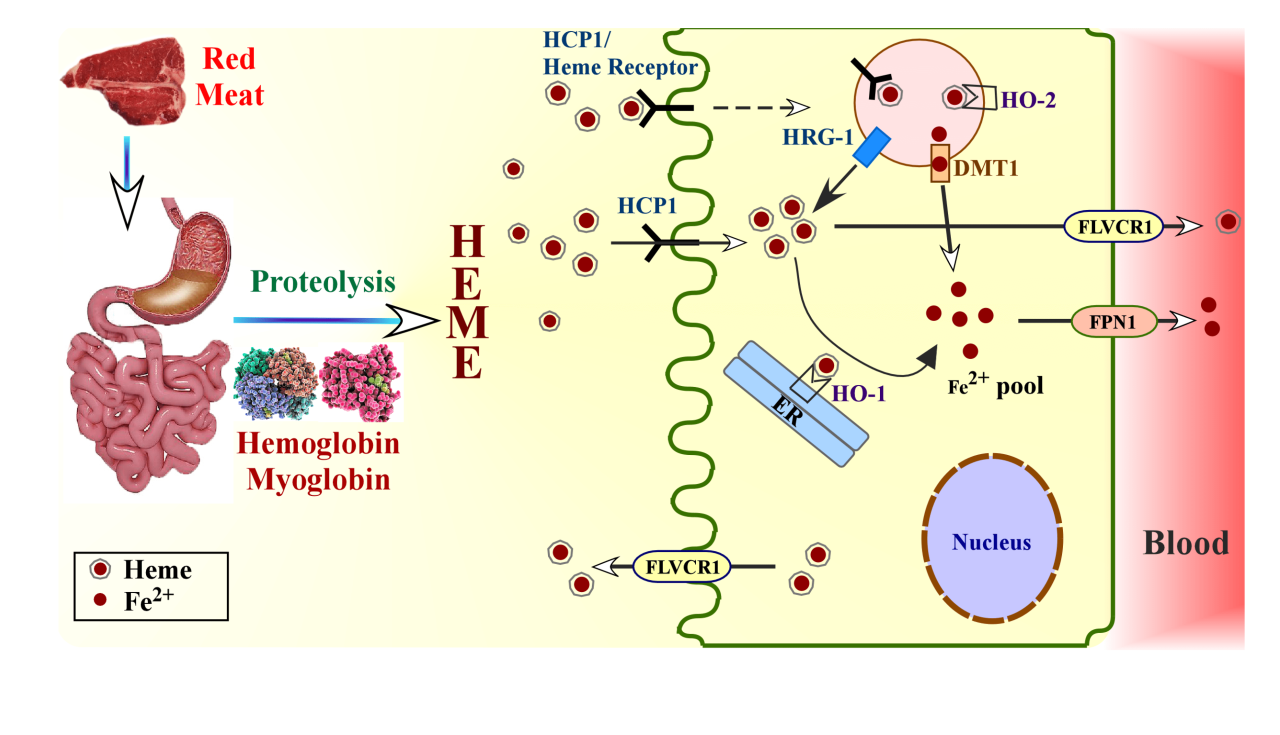
Heme Degradation
Heme degradation is a crucial process in the body that involves the breakdown of heme, the iron-containing porphyrin found in hemoglobin and other hemoproteins. This process plays a vital role in maintaining iron homeostasis and eliminating potentially toxic heme metabolites.
Role of Heme Oxygenase Enzymes
The degradation of heme is initiated by heme oxygenase enzymes (HOs), which are microsomal enzymes that catalyze the oxidative cleavage of the heme ring. There are three isoforms of HO: HO-1, HO-2, and HO-3. HO-1 is the inducible isoform that is upregulated in response to various stress conditions, such as oxidative stress and inflammation.
HO-2 is the constitutive isoform that is expressed at a basal level in most tissues. HO-3 is a recently discovered isoform that is mainly expressed in the brain and testes.
Formation and Significance of Biliverdin and Bilirubin
The initial product of heme degradation by HOs is biliverdin, a green pigment. Biliverdin is then reduced to bilirubin, a yellow pigment, by biliverdin reductase. Bilirubin is water-insoluble and must be conjugated with glucuronic acid to become water-soluble and excretable.
This conjugation process occurs in the liver and is catalyzed by the enzyme UDP-glucuronosyltransferase (UGT1A1).
Biliverdin and bilirubin are significant heme degradation products because they are responsible for the characteristic color of bile and feces. Bilirubin levels in the blood can also be used as a diagnostic marker for liver disease, as elevated bilirubin levels can indicate liver dysfunction or obstruction of the biliary system.
Clinical Implications of Heme Degradation Disorders
Disorders of heme degradation can lead to a variety of clinical conditions. One common disorder is hemolytic anemia, which is characterized by the excessive destruction of red blood cells and the release of heme into the bloodstream. This can lead to increased bilirubin levels and jaundice, as well as anemia.
Another disorder is porphyria, which is a group of metabolic disorders that result from defects in the heme biosynthesis pathway. Porphyrias can cause a variety of symptoms, including skin photosensitivity, abdominal pain, and neurological problems.
Heme-Protein Interactions: Complete The Sentences About Heme
Heme interacts with various proteins to perform diverse biological functions. These interactions range from covalent binding to non-covalent associations.
Covalent Binding
Covalent binding involves the formation of a chemical bond between heme and the protein. This type of interaction is found in:
- Cytochromes:Heme is covalently bound to cysteine residues in cytochromes, which are electron carriers in cellular respiration.
- Catalase:Heme is covalently attached to a histidine residue in catalase, an enzyme that decomposes hydrogen peroxide.
Non-Covalent Binding
Non-covalent interactions include hydrogen bonding, hydrophobic interactions, and electrostatic forces. These interactions allow for dynamic heme binding and are found in:
- Hemoglobin:Heme is non-covalently bound to globin proteins in hemoglobin, the oxygen-carrying molecule in red blood cells.
- Myoglobin:Heme is also non-covalently bound to globin proteins in myoglobin, which stores oxygen in muscle tissue.
Role in Protein Folding and Stability
Heme binding can influence protein folding and stability. The hydrophobic nature of heme can promote protein folding and prevent aggregation. Additionally, the coordination of heme with amino acid side chains can stabilize protein structure.
Heme in Oxygen Transport and Metabolism
Heme is an essential component of many proteins involved in oxygen transport and metabolism. In particular, heme plays a crucial role in the oxygen transport proteins hemoglobin and myoglobin. Hemoglobin is found in red blood cells and is responsible for carrying oxygen from the lungs to the rest of the body.
Myoglobin is found in muscle cells and stores oxygen for use during periods of high demand.
Mechanism of Oxygen Binding and Release
Heme proteins bind oxygen through a process called cooperative binding. In cooperative binding, the binding of one oxygen molecule to a heme protein increases the affinity of the remaining heme groups for oxygen. This allows heme proteins to bind and release oxygen in a controlled manner, ensuring that oxygen is delivered to tissues when it is needed.
Importance in Cellular Respiration
Heme is also essential for cellular respiration, the process by which cells generate energy. Heme is a component of cytochrome c, a protein that is involved in the electron transport chain. The electron transport chain is a series of proteins that pass electrons from one to another, ultimately generating ATP, the energy currency of the cell.
Heme in Signal Transduction
Heme is a versatile molecule that not only plays a crucial role in oxygen transport and metabolism but also acts as a signaling molecule in cellular processes. Its ability to bind to specific proteins and modulate their activity makes it a key regulator of various signaling pathways.
Mechanisms of Heme Signaling
Heme exerts its signaling effects through several mechanisms:
- Direct Binding to Enzymes:Heme can bind directly to enzymes and alter their activity. For example, heme binding to the enzyme heme oxygenase-1 (HO-1) enhances its enzymatic activity, leading to the production of carbon monoxide (CO) and biliverdin, which have anti-inflammatory and antioxidant effects.
- Regulation of Gene Expression:Heme can also regulate gene expression by binding to transcription factors and influencing their DNA binding affinity. The heme-regulated inhibitor kinase (HRI) is a transcription factor that binds heme and represses the expression of genes involved in iron metabolism.
Therapeutic Applications
Targeting heme signaling pathways holds promising therapeutic potential for various diseases:
- Anti-inflammatory Effects:Heme oxygenase-1 (HO-1) is a key enzyme in heme catabolism, and its induction has been shown to have anti-inflammatory effects in models of sepsis, arthritis, and other inflammatory diseases.
- Antioxidant Effects:Heme oxygenase-1 also produces carbon monoxide (CO), which has antioxidant and anti-apoptotic properties. Targeting HO-1 induction could be a potential therapeutic strategy for oxidative stress-related diseases.
- Cancer Treatment:Heme signaling has been implicated in cancer cell growth and survival. Targeting heme metabolism and signaling pathways could provide novel therapeutic approaches for cancer treatment.
Heme in Disease
Heme, the iron-containing porphyrin prosthetic group, plays a crucial role in various biological processes. However, its dysregulation can contribute to the pathogenesis of several diseases.
Anemia, characterized by reduced red blood cell count or hemoglobin content, can result from impaired heme biosynthesis or excessive heme degradation. Iron deficiency, genetic defects in heme synthesis enzymes, and certain chronic diseases can lead to anemia.
Porphyriasare a group of metabolic disorders caused by defects in heme biosynthesis. These defects lead to the accumulation of heme precursors, known as porphyrins, which can cause photosensitivity, neurological symptoms, and skin problems.
Neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, have been linked to heme dysregulation. Heme can promote oxidative stress and neuronal damage, contributing to the progression of these diseases.
Mechanisms of Heme-Induced Tissue Damage
Heme can contribute to tissue damage through several mechanisms:
- Oxidative stress: Heme can undergo redox reactions, generating reactive oxygen species (ROS) that can damage cellular components, including lipids, proteins, and DNA.
- Lipid peroxidation: Heme can catalyze the peroxidation of lipids, leading to the formation of lipid hydroperoxides that can disrupt membrane integrity and function.
- Protein denaturation: Heme can interact with and denature proteins, altering their structure and function.
- Mitochondrial dysfunction: Heme can accumulate in mitochondria, impairing mitochondrial function and contributing to cell death.
Therapeutic Strategies Targeting Heme in Disease
Therapeutic strategies targeting heme in disease aim to either reduce heme levels or mitigate its toxic effects:
- Heme oxygenase inducers: Drugs that induce heme oxygenase, an enzyme that degrades heme, can reduce heme levels and protect against heme-induced tissue damage.
- Antioxidants: Antioxidants can neutralize ROS generated by heme, preventing oxidative stress and tissue damage.
- Iron chelators: Iron chelators can bind to heme and prevent its interaction with cellular components, reducing its toxic effects.
General Inquiries
What is the significance of the axial ligands in heme’s coordination sphere?
The axial ligands play a crucial role in stabilizing the heme structure and modulating its reactivity. They influence the electronic properties of the iron ion, affecting its oxidation state and coordination geometry.
How does heme contribute to cellular respiration and energy production?
Heme serves as the prosthetic group in proteins like cytochrome c and cytochrome oxidase, which are essential components of the electron transport chain. It facilitates the transfer of electrons, enabling the efficient production of ATP, the cellular energy currency.
What are the potential therapeutic applications of targeting heme signaling pathways?
Targeting heme signaling pathways holds promise for treating various diseases, including cancer and neurodegenerative disorders. By modulating heme levels or interfering with its interactions with signaling molecules, researchers aim to develop novel therapeutic strategies.