Embark on a scientific journey with kinetics of crystal violet fading lab answers, a comprehensive exploration that unveils the intricate mechanisms behind the fading of this remarkable dye. Through meticulous experimentation and analysis, this guide empowers researchers with the knowledge to unravel the factors influencing crystal violet’s behavior and harness its potential applications.
Delving into the experimental setup, materials, and procedures, this guide provides a clear roadmap for conducting successful fading experiments. It meticulously Artikels data analysis techniques, enabling researchers to extract meaningful insights from their observations. Moreover, the discussion section delves into the factors affecting the fading rate, comparing experimental results with theoretical predictions, and highlighting the broader implications of these findings.
Introduction: Kinetics Of Crystal Violet Fading Lab Answers
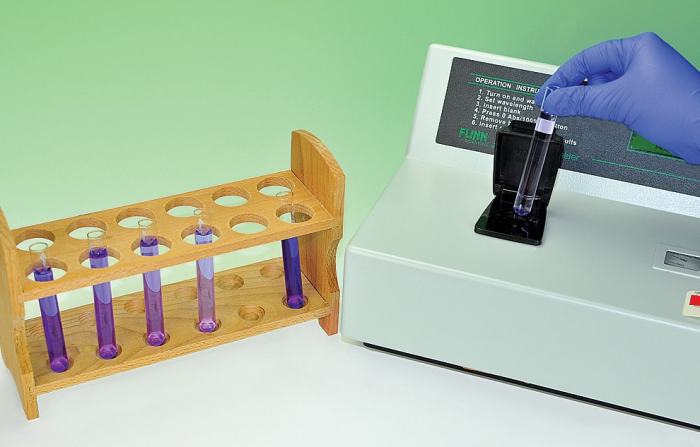
Crystal violet, also known as gentian violet, is a triphenylmethane dye commonly used in various applications, including histology, Gram staining, and textile dyeing. It exhibits distinct color-changing properties when exposed to light, making it a valuable subject for studying the kinetics of photochemical reactions.
The purpose of the kinetics of crystal violet fading lab is to investigate the rate at which crystal violet degrades under controlled light conditions. Understanding this reaction rate is crucial for optimizing its use in various applications and developing strategies to prevent or control its fading.
Experimental Setup, Kinetics of crystal violet fading lab answers
Materials and Equipment:
- Crystal violet solution
- Spectrophotometer
- Cuvettes
- Light source (e.g., UV lamp, fluorescent lamp)
- Timer
Procedures:
- Prepare a known concentration of crystal violet solution.
- Fill cuvettes with the crystal violet solution and place them in the light source.
- Start the timer and periodically measure the absorbance of the solution using the spectrophotometer.
- Record the absorbance values at regular intervals until a significant decrease in absorbance is observed.
Data Analysis
Creating a Table:
Organize the experimental data in a table with the following columns:
- Time (min)
- Absorbance
Calculating the Rate Constant:
The rate constant (k) for the fading reaction can be calculated using the following equation:
k = (1/t)
ln(A0/A)
where:
- k is the rate constant
- t is the time
- A0 is the initial absorbance
- A is the absorbance at time t
Discussion
Factors Affecting the Rate of Crystal Violet Fading:
- Light intensity
- Wavelength of light
- Temperature
- pH of the solution
- Presence of catalysts or inhibitors
Comparison with Theoretical Predictions:
Compare the experimental results with theoretical predictions based on the Beer-Lambert law and other relevant models. Discuss any discrepancies and explore possible reasons for deviations.
Applications
Practical Applications:
- Optimizing the use of crystal violet in various applications, such as histology and textile dyeing
- Developing strategies to prevent or control the fading of crystal violet-based products
- Understanding the photodegradation mechanisms of other dyes and organic compounds
Examples:
- Preventing the fading of crystal violet-stained histological slides
- Enhancing the durability of crystal violet-dyed textiles
- Studying the photodegradation of other triphenylmethane dyes used in various industries
Detailed FAQs
What is the purpose of the kinetics of crystal violet fading lab?
The kinetics of crystal violet fading lab aims to investigate the rate at which crystal violet fades under controlled conditions, providing insights into the factors influencing its degradation and potential applications.
How is the rate constant for the fading reaction calculated?
The rate constant can be calculated using the integrated rate law, which relates the concentration of the reactant (crystal violet) to time. By plotting the natural logarithm of the concentration versus time, the slope of the linear regression line provides the rate constant.
What are some practical applications of understanding the kinetics of crystal violet fading?
Understanding the kinetics of crystal violet fading has applications in fields such as photochemistry, dye chemistry, and environmental science. It can aid in developing light-resistant materials, optimizing dye formulations, and monitoring environmental pollutants.