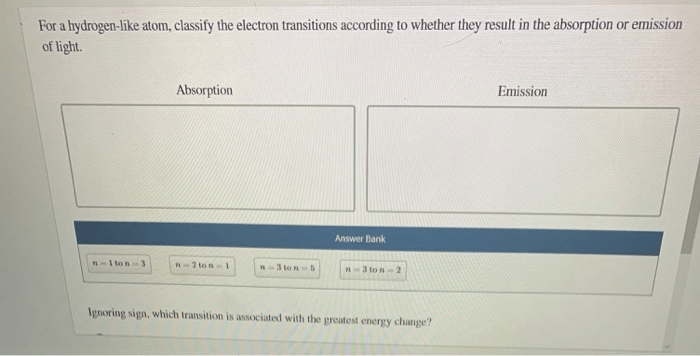
For a hydrogen like atom classify the electron transitions – In the realm of quantum mechanics, electron transitions play a pivotal role in shaping the properties of atoms. For a hydrogen-like atom, classifying electron transitions provides a fundamental understanding of its energy level structure and behavior. This article delves into the intricacies of electron transitions, their classification, and their diverse applications.
A hydrogen-like atom, possessing a single electron orbiting a nucleus, serves as a paradigm for studying electron transitions. The unique characteristics of this atom enable the precise classification of transitions based on their energy changes and wavelengths.
Hydrogen Atom Overview
A hydrogen atom is the simplest atom, consisting of a single proton and a single electron. It is the most abundant element in the universe, accounting for about 75% of all matter.
The electron in a hydrogen atom occupies one of a series of discrete energy levels, which are determined by the quantum mechanical properties of the atom. The lowest energy level, known as the ground state, has an energy of -13.6 eV.
The next higher energy level, known as the first excited state, has an energy of -3.4 eV. Higher energy levels exist as well.
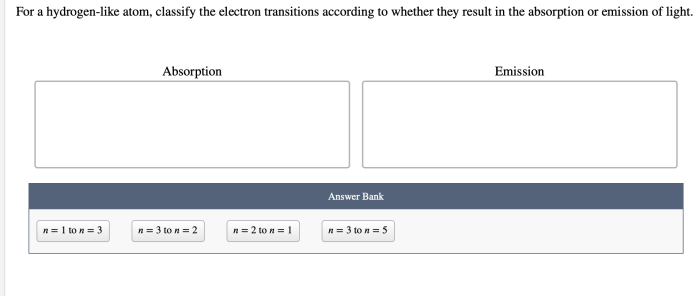
Electron Transitions in Hydrogen-like Atoms

Electron transitions are changes in the energy level of an electron in an atom. They can occur when an electron absorbs or emits a photon of light. The energy of the photon must be equal to the difference in energy between the two energy levels involved in the transition.
The factors that influence electron transitions include the following:
- The energy of the incoming photon
- The energy levels of the atom
- The selection rules for electron transitions
Energy Absorption and Emission, For a hydrogen like atom classify the electron transitions
When an electron absorbs a photon of light, it transitions to a higher energy level. This process is known as excitation. When an electron emits a photon of light, it transitions to a lower energy level. This process is known as de-excitation.
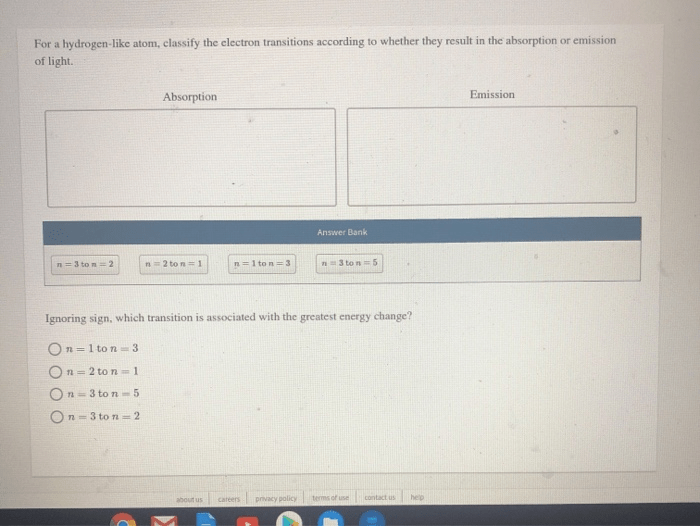
Classification of Electron Transitions
Electron transitions in hydrogen-like atoms can be classified into three types:
- Electric dipole transitions
- Magnetic dipole transitions
- Quadrupole transitions
Electric dipole transitions are the most common type of electron transition. They occur when the electron changes its orbital angular momentum by one unit. Magnetic dipole transitions occur when the electron changes its spin angular momentum by one unit. Quadrupole transitions occur when the electron changes its orbital angular momentum by two units.
The following table summarizes the different types of electron transitions in hydrogen-like atoms:
| Transition Type | Energy Change | Wavelength |
|---|---|---|
| Electric dipole | ΔE = hf | λ = hc/ΔE |
| Magnetic dipole | ΔE = 2μBB | λ = hc/2μBB |
| Quadrupole | ΔE = 3e2q2/4I | λ = hc/3e2q2/4I |
Applications of Electron Transitions: For A Hydrogen Like Atom Classify The Electron Transitions
Electron transitions have a wide range of applications in various fields, including spectroscopy and astrophysics.
In spectroscopy, electron transitions are used to determine the composition and properties of materials. For example, atomic absorption spectroscopy is used to measure the concentration of metals in a sample. In astrophysics, electron transitions are used to study the composition and properties of stars and other celestial objects.
Query Resolution
What is the significance of electron transitions in hydrogen-like atoms?
Electron transitions govern the absorption and emission of energy, providing insights into the atom’s energy level structure and properties.
How are electron transitions classified?
Transitions are classified based on their energy change and wavelength, resulting in categories such as absorption, emission, and resonance transitions.
What are the applications of classifying electron transitions?
This classification aids in the identification of elements, the analysis of stellar spectra, and the determination of material composition.