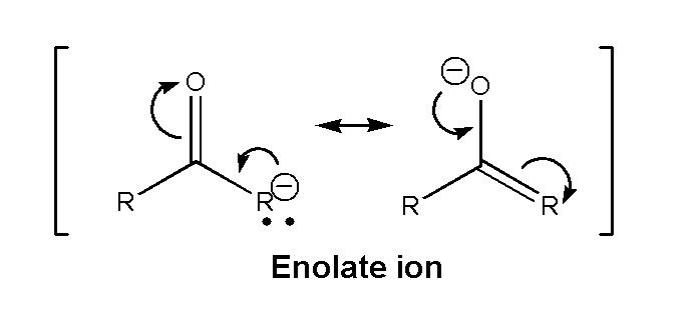
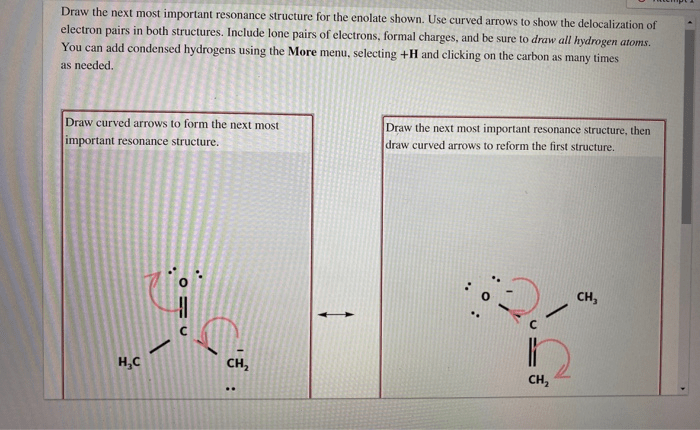
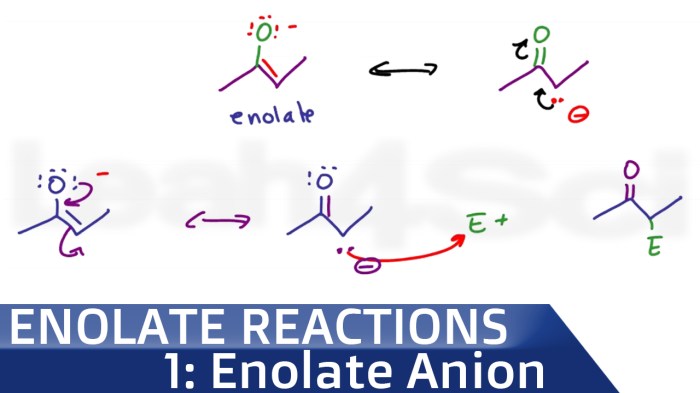
Draw the resonance structure of the enolate ion is an essential skill for organic chemists. It allows us to understand the electronic structure and reactivity of this important intermediate. In this guide, we will provide a step-by-step procedure for drawing the resonance structures of the enolate ion and discuss the factors that affect their stability.
Resonance structures are Lewis structures that represent the different ways in which the electrons in a molecule can be arranged. The enolate ion is a resonance hybrid of two resonance structures, which means that its electronic structure is a weighted average of these two structures.
Resonance Structures of the Enolate Ion
The enolate ion is a resonance hybrid, meaning that it can be represented by multiple Lewis structures. This resonance results from the delocalization of the negative charge over the carbon and oxygen atoms. The resonance structures of the enolate ion are important because they help to explain its stability and reactivity.To
draw the resonance structures of the enolate ion, start by drawing the Lewis structure of the starting ketone or aldehyde. Then, remove a proton from the alpha carbon and add a negative charge to the oxygen atom. This will create two resonance structures, one with the negative charge on the carbon atom and one with the negative charge on the oxygen atom.
Stability of Resonance Structures
The stability of resonance structures depends on several factors, including the number of contributing structures, the electronegativity of the atoms involved, and the resonance energy. The more contributing structures a resonance hybrid has, the more stable it is. The more electronegative the atoms involved in the resonance, the more stable the resonance hybrid.
And the greater the resonance energy, the more stable the resonance hybrid.The enolate ion has two resonance structures, which are equally stable. This is because the negative charge is delocalized over two electronegative atoms, carbon and oxygen. The resonance energy of the enolate ion is also high, which contributes to its stability.
Applications of Enolate Ion Resonance Structures, Draw the resonance structure of the enolate ion
The resonance structures of the enolate ion are important in organic chemistry because they help to explain its reactivity. For example, the enolate ion can react with electrophiles at either the carbon or oxygen atom. The regioselectivity of these reactions can be predicted based on the resonance structures of the enolate ion.The
resonance structures of the enolate ion can also be used to explain its acidity. The enolate ion is a weak acid, with a pKa of around 15. This is because the negative charge on the enolate ion is delocalized, which makes it less likely to donate a proton.
Advanced Concepts
The resonance structures of the enolate ion can be explained using molecular orbital theory. The enolate ion has a delocalized pi system, which consists of the p orbitals on the carbon and oxygen atoms. The negative charge on the enolate ion is delocalized over this pi system, which results in the resonance structures.The
resonance structures of the enolate ion can also be used to explain its relationship to the pKa of the enolate ion. The more stable the resonance structures, the lower the pKa of the enolate ion. This is because the more stable the resonance structures, the less likely the enolate ion is to donate a proton.
Query Resolution: Draw The Resonance Structure Of The Enolate Ion
What is an enolate ion?
An enolate ion is a resonance hybrid of two resonance structures, which means that its electronic structure is a weighted average of these two structures.
How do you draw the resonance structures of an enolate ion?
To draw the resonance structures of an enolate ion, you need to first identify the atoms that are involved in the resonance. The enolate ion is a resonance hybrid of two resonance structures, which means that its electronic structure is a weighted average of these two structures.
What are the factors that affect the stability of resonance structures?
The stability of resonance structures is affected by a number of factors, including the number of contributing structures, the electronegativity of the atoms involved, and the resonance energy.