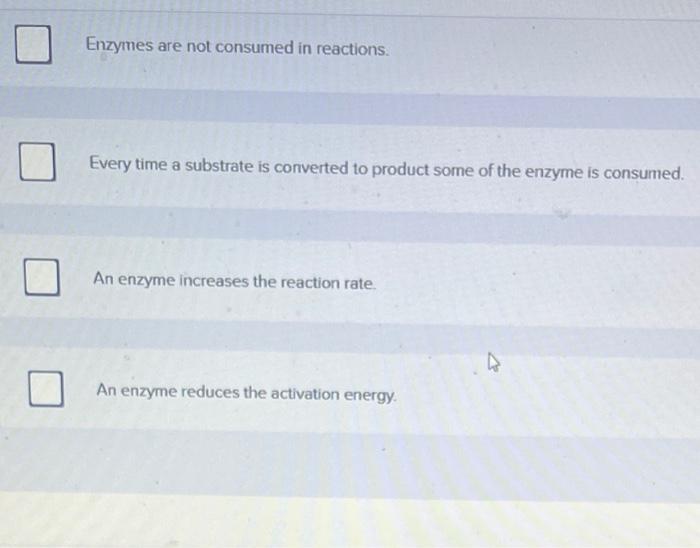
Which of the following is true concerning enzymes? This question delves into the fascinating realm of enzymes, the molecular catalysts that orchestrate countless biological processes. From their intricate structure and diverse functions to the factors influencing their activity and the mechanisms governing their regulation, this comprehensive exploration unravels the enigmatic world of enzymes.
Enzymes, the workhorses of cellular machinery, facilitate biochemical reactions with remarkable efficiency and specificity. Their intricate structure, composed of amino acid chains folded into precise conformations, provides the foundation for their catalytic prowess. Each enzyme exhibits a unique active site, a molecular pocket that binds to specific substrates, enabling the precise execution of chemical transformations.
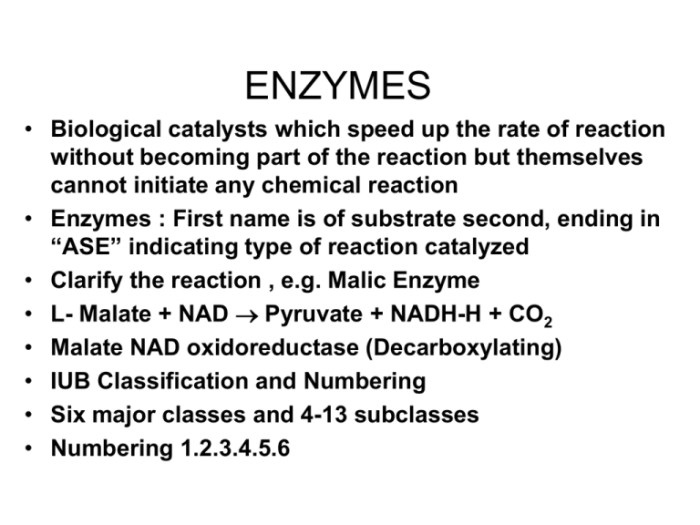
1. Definition and Nature of Enzymes
Enzymes are biological catalysts that accelerate chemical reactions in living organisms. They are highly specific proteins that interact with specific substrates and lower the activation energy required for a reaction to occur.
Enzymes are composed of amino acids arranged in a unique three-dimensional structure. This structure creates an active site, where the substrate binds and the catalytic reaction takes place.
Examples of Enzymes and Their Functions
- Amylase: Breaks down starch into sugars
- Protease: Breaks down proteins into amino acids
- Lipase: Breaks down fats into fatty acids and glycerol
2. Enzyme Activity and Factors Affecting It: Which Of The Following Is True Concerning Enzymes
Enzyme activity is influenced by various factors, including:
Temperature
Enzymes have an optimal temperature range at which they exhibit maximum activity. Deviations from this range can lead to decreased activity or denaturation.
pH
Enzymes also have an optimal pH range for activity. Changes in pH can alter the ionization of the enzyme and substrate, affecting their interaction and catalytic efficiency.
Substrate Concentration
The rate of enzyme-catalyzed reactions increases with increasing substrate concentration until a saturation point is reached.
Enzyme Kinetics and the Michaelis-Menten Equation
Enzyme kinetics describes the mathematical relationship between enzyme activity and substrate concentration. The Michaelis-Menten equation quantifies this relationship and provides insights into enzyme-substrate interactions.
Cofactors and Coenzymes
Some enzymes require additional molecules called cofactors or coenzymes to function properly. Cofactors are metal ions, while coenzymes are organic molecules that participate in the catalytic reaction.
3. Enzyme Inhibition
Enzyme inhibitors are molecules that reduce enzyme activity. They can be classified into three main types:
Competitive Inhibition
Competitive inhibitors bind to the active site of the enzyme, competing with the substrate for binding. This decreases the rate of reaction.
Non-competitive Inhibition
Non-competitive inhibitors bind to a site on the enzyme other than the active site. This changes the enzyme’s conformation and reduces its catalytic activity.
Uncompetitive Inhibition
Uncompetitive inhibitors bind to the enzyme-substrate complex, causing a conformational change that decreases the rate of reaction.
Mechanisms of Action of Enzyme Inhibitors
Enzyme inhibitors can act by blocking the active site, altering the enzyme’s conformation, or interfering with cofactor binding.
Examples of Enzyme Inhibitors and Their Applications
Enzyme inhibitors have various applications in medicine, research, and industry, such as antibiotics, antiviral drugs, and pesticides.
4. Enzyme Regulation
Enzyme regulation is crucial for controlling metabolic pathways and maintaining cellular homeostasis.
Allosteric Regulation
Allosteric regulation involves the binding of regulatory molecules to allosteric sites on the enzyme, affecting its activity.
Covalent Modification
Covalent modification involves the addition or removal of chemical groups to or from the enzyme, altering its activity.
Gene Expression, Which of the following is true concerning enzymes
Enzyme activity can also be regulated at the level of gene expression, by controlling the synthesis or degradation of the enzyme.
Importance of Enzyme Regulation in Cellular Metabolism
Enzyme regulation ensures that metabolic pathways operate efficiently and respond to changes in cellular conditions.
Examples of Enzyme Regulation in Different Biological Processes
Enzyme regulation plays a critical role in processes such as glycolysis, gluconeogenesis, and the citric acid cycle.
5. Applications of Enzymes in Biotechnology
Enzymes have numerous applications in biotechnology, including:
Food Processing
Enzymes are used in food processing to improve flavor, texture, and nutritional value.
Pharmaceuticals
Enzymes are used in the production of antibiotics, vitamins, and other pharmaceuticals.
Biofuels
Enzymes are used to convert biomass into biofuels, such as ethanol and biodiesel.
Advantages and Limitations of Using Enzymes in Biotechnology
Enzymes offer advantages such as high specificity and efficiency. However, they can be expensive and susceptible to denaturation.
Examples of Specific Enzyme Applications and Their Impact on Industries
Specific examples of enzyme applications include the use of proteases in detergents and amylases in baking.
FAQ Summary
What is the primary function of enzymes?
Enzymes act as catalysts, facilitating biochemical reactions by lowering the activation energy required for the reaction to occur.
How does temperature affect enzyme activity?
Temperature can significantly influence enzyme activity, with an optimal temperature range for each enzyme. Deviations from this range can lead to decreased activity or even denaturation.
What is the difference between competitive and non-competitive enzyme inhibition?
Competitive inhibitors bind to the active site of an enzyme, competing with the substrate for binding. Non-competitive inhibitors bind to a different site on the enzyme, causing conformational changes that impair its catalytic activity.