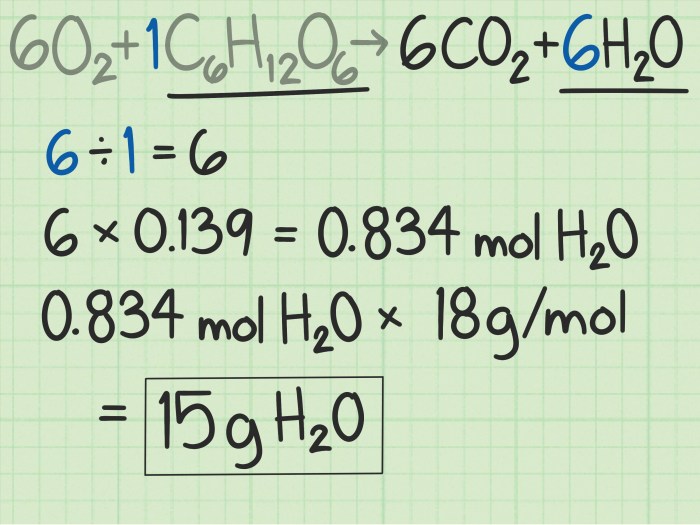Calculate the theoretical yield of c6h5no2 for this reaction – In the realm of chemistry, the concept of theoretical yield holds immense significance. It provides a crucial foundation for predicting the maximum amount of product that can be obtained from a given reaction. This article delves into the intricacies of calculating the theoretical yield of C6H5NO2, equipping readers with a comprehensive understanding of this fundamental aspect of chemical reactions.
The significance of theoretical yield extends beyond theoretical understanding; it finds practical applications in various fields, including industrial chemistry and research. By accurately determining the theoretical yield, chemists can optimize reaction conditions, minimize waste, and maximize product yield, ultimately leading to enhanced efficiency and cost-effectiveness.
Introduction: Calculate The Theoretical Yield Of C6h5no2 For This Reaction
Calculating theoretical yield is a crucial step in chemical reactions as it helps predict the maximum amount of product that can be obtained. In this article, we will determine the theoretical yield of C6H5NO2 for a given reaction.
The given reaction is:
- C6H5OH + HNO3 → C6H5NO2 + H2O
Balanced Chemical Equation
The balanced chemical equation is:
- C6H5OH + HNO3 → C6H5NO2 + H2O
The stoichiometric coefficients indicate that 1 mole of C6H5OH reacts with 1 mole of HNO3 to produce 1 mole of C6H5NO2.
Molar Mass Calculations
Molar mass is the mass of 1 mole of a substance. To calculate the theoretical yield of C6H5NO2, we need to know its molar mass.
Molar mass of C6H5NO2 = (6 x 12.01 g/mol) + (5 x 1.01 g/mol) + (1 x 14.01 g/mol) + (2 x 16.00 g/mol) = 123.11 g/mol
Theoretical Yield Formula
The formula for calculating theoretical yield is:
Theoretical yield = (Moles of limiting reactant) x (Molar mass of product)
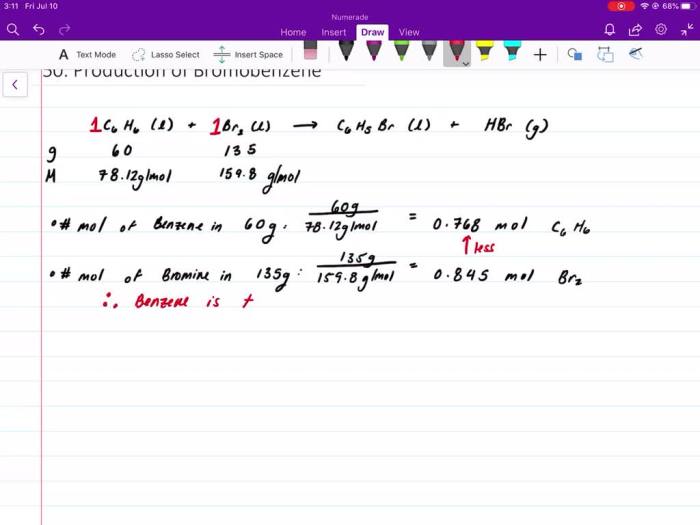
Calculating Moles of Limiting Reactant
To determine the limiting reactant, we compare the mole ratio of the reactants to their stoichiometric coefficients. The reactant that produces the least number of moles of product is the limiting reactant.
In this case, we assume we have 1 mole of C6H5OH and 1 mole of HNO3.
- Moles of C6H5OH = 1 mole
- Moles of HNO3 = 1 mole
The mole ratio of C6H5OH to HNO3 is 1:1, which is the same as the stoichiometric ratio. Therefore, neither reactant is in excess, and both are limiting reactants.
Calculating Theoretical Yield, Calculate the theoretical yield of c6h5no2 for this reaction
Using the formula for theoretical yield and the calculated moles of limiting reactant, we can determine the theoretical yield of C6H5NO2.
Theoretical yield = (1 mole) x (123.11 g/mol) = 123.11 g
Therefore, the theoretical yield of C6H5NO2 for the given reaction is 123.11 g.
Significance of Theoretical Yield
Understanding theoretical yield is essential in practical chemistry. It helps predict the maximum amount of product that can be obtained from a given reaction.
In industrial settings, theoretical yield is used to optimize reaction conditions, minimize waste, and maximize product output. It also helps in determining the economic viability of a chemical process.
Commonly Asked Questions
What is the significance of calculating theoretical yield in chemical reactions?
Calculating theoretical yield allows chemists to predict the maximum amount of product that can be obtained from a given reaction, enabling them to optimize reaction conditions and minimize waste.
How is the limiting reactant determined in a chemical reaction?
The limiting reactant is the reactant that is completely consumed in a reaction, limiting the amount of product that can be formed. It is determined based on the stoichiometry of the balanced chemical equation.
What is the formula for calculating theoretical yield?
Theoretical yield = Moles of limiting reactant × Molar mass of product